PREZISTA®/r: Tolerability Proven Over Time
Selected laboratory abnormalities1, 2
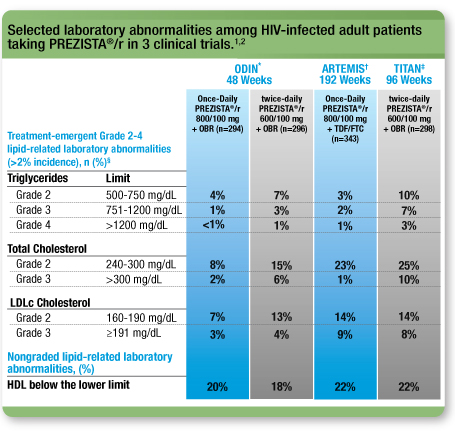
This is not a complete list of all laboratory abnormalities.
*
ODIN: A randomized, open-label, Phase 3, 48-week, noninferiority clinical trial comparing Once-Daily PREZISTA®/ritonavir 800/100 mg with twice-daily PREZISTA®/ritonavir 600/100 mg in treatment-experienced adult patients with no DRV RAMs (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V).
†
ARTEMIS: A randomized, controlled, open-label, Phase 3, noninferiority clinical trial comparing PREZISTA®/r 800/100 mg once daily (n=343) with Kaletra® 800/200 mg per day (n=346) in treatment-naïve adult patients with HIV-1
RNA ≥5000 copies/mL.
‡
TITAN: A randomized, controlled, open-label, Phase 3 clinical trial comparing PREZISTA®/r 600/100 mg twice daily (n=298) with Kaletra 400/100 mg twice daily (n=297) in less treatment-experienced, lopinavir-naïve adults.
§
Based on the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Paediatric Adverse Events 2004, which does not have a grade 1 classification for triglycerides and grade 4 for total cholesterol and LDL.
Kaletra (lopinavir/ritonavir) is a registered trademark of AbbVie Inc.
OBR=optimized background regimen; TDF/FTC=tenofovir disoproxil fumarate/emtricitabine; LDL=low-density lipoprotein; HDL=high-density lipoprotein;



