PREZISTA®/r: Tolerability Proven Over Time
Low discontinuation due to ADRs* with Once-Daily PREZISTA®/r in the first 90 days and through 192 weeks1, 2
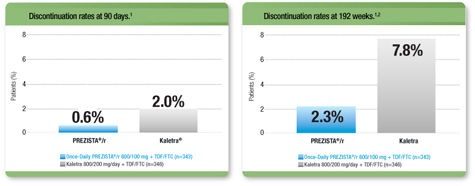
* Excluding laboratory abnormalities as ADRs.
ADRs=adverse drug reactions; TDF/FTC=tenofovir disoproxil fumarate/emtricitabine.
Kaletra (lopinavir/ritonavir) is a registered trademark of AbbVie Inc.



