PREZISTA® /r: Once Daily vs Twice Daily
ODIN* Trial
A Comparative Trial of Once-Daily PREZISTA®/r vs Twice-Daily PREZISTA®/r in Treatment-Experienced Adult Patients With No DRV RAMs†1-3
Study design1, 2
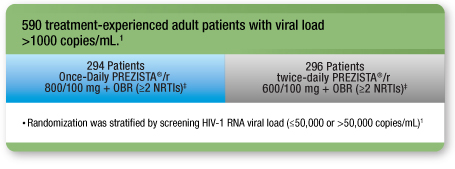
- A 48-week randomized, open-label, noninferiority, clinical trial in treatment-experienced adult patients with no DRV RAMs comparing Once-Daily PREZISTA®/ritonavir 800/100 mg with twice-daily PREZISTA®/ritonavir 600/100 mg1
Primary end point
- Demonstrate noninferiority of Once-Daily PREZISTA®/r 800/100 mg vs twice-daily PREZISTA®/r 600/100 mg1
Primary efficacy parameter
- Virologic response (HIV-1 RNA <50 copies/mL) at Week 48
- Noninferiority was determined using a 12% margin (delta): the lower boundary of the 95% confidence interval of the difference in virologic response between Once-Daily PREZISTA®/r and twice-daily PREZISTA®/r could not cross -12%1
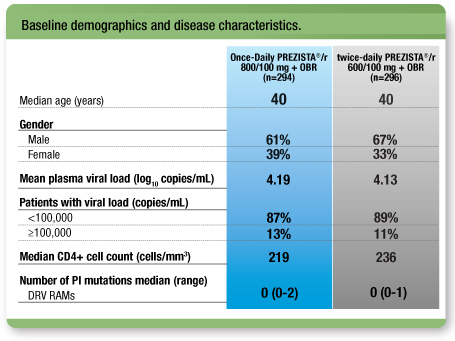
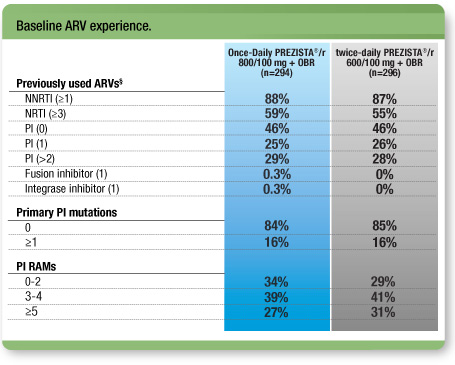
Treatment arms were well balanced1
- 54% of all patients had previously taken ≥1 PIs2
- The median duration of treatment with PIs was 42.4 months
* Once-daily Darunavir In Treatment-Experie Nced Patients.
† DRV RAMs=darunavir resistance-associated mutations; patients with screening genotype results showing no DRV RAMs, which included the following mutations: V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, and L89V.
‡ Investigator-selected OBR based on ARV history and resistance testing.
§ The most common previously used PIs were lopinavir (26%) and indinavir (21%). Eighty-one percent (81%) of subjects used boosted PIs compared with 27% of subjects who used unboosted PIs.
NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; PI RAMs=protease inhibitor resistance-associated mutations.






